Technology expands upon quantitative phase imaging, which can be integrated into microscopes commonly found in the laboratory – digital holographic microscopy with diode pumped lasers (enhanced Raman and lasers for bioimaging).
In recent years, quantitative phase imaging (QPI) has been continuously improved for purposes of high-resolution, label-free quantitative microscopy1. Label-free imaging has received increased attention in the last decade as a minimally invasive way to observe proteins and cells, and to study the behavior and properties of biological specimens with minimized modification and sample preparation. Using noninvasive techniques means samples under investigation are not influenced by fluorophores or dyes, which can change the physiological processes or behavior of samples by causing cellular motility or migration. Moreover, because QPI requires only low light intensities for object illumination, the potential harm light could cause to a sample is minimized. Low light is a precondition for the long-term monitoring of living cells because high doses of light radiation can cause cell death or photo damage.
Digital holographic microscopy (DHM)2,3 is an interferometry-based variant of QPI that typically uses a laser as a coherent light source and provides QPI by detecting specimen-induced optical path length changes against the surrounding environment. DHM can be modularly integrated into common optical microscopes3,4, which allows its integration as a label-free imaging modality in research laboratories.
In DHM, reconstruction of digitally captured holograms is performed numerically by a computer. Thus, multifocus imaging of specimen parts in various layers is achieved — and even subsequent autofocusing from single captured digital holograms — without the need for mechanical focus realignment5. This is advantageous for the correction of unpredictable focus drifts during long-term observations of living cells, or for imaging of cell movements in 3D environments such as collagen or gel matrices. In addition, DHM-based phase contrast imaging can simplify automated object tracking and image segmentation for quantification of cell migration and morphology by extraction of absolute biophysical parameters such as cellular volume, thickness, and dry mass1. Moreover, the evaluation of quantitative DHM phase images provides access to optical parameters such as the cellular refractive index, which is related to the cellular water content, intracellular solute concentrations, and tissue density3,6. This qualifies DHM as a viable label-free cytometry tool for the identification and characterization of tumors, blood, and stem cells in mixtures or composites; for time-lapse in vitro toxicity and drug testing; and for ex vivo histology of dissected tissues.
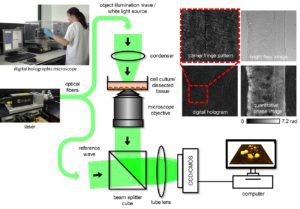 Figure 1. The concept of laser-based inverted off-axis DHM in transmission configuration for correlated label-free bright-field and quantitative phase imaging of dissected tissues (upper right) and living cell cultures in the environment of a biomedical laboratory. Here showing the Cobolt 06-DPL 532 nm diode pumped laser with long coherence length.
Figure 1. The concept of laser-based inverted off-axis DHM in transmission configuration for correlated label-free bright-field and quantitative phase imaging of dissected tissues (upper right) and living cell cultures in the environment of a biomedical laboratory. Here showing the Cobolt 06-DPL 532 nm diode pumped laser with long coherence length.
Read the most recent editorial from one of our reference customers using the 06-DPL 532 nm:
‚Digital Holographic Microscopy enhances cytometry and histology‘ by Dr Björn Kemper from BIOMEDICAL TECHNOLOGY CENTER of University of Münster, Germany, in Europhotonics Autumn edition

