Cobolt SkyraTM is an extremely compact, permanently aligned and service free multi-line laser platform with up to 4 laser lines integrated in a single hand sized and hermetically sealed package with integrated electronics.
Conventional laser combiner solutions used in fluorescence-based bio-instrumentation equipment use multiple individual lasers combined through optical elements in systems that are bulky, costly to manufacture and challenging to keep aligned.
All optical elements are assembled onto one temperature-controlled platform and components for beam shaping and alignment are integral parts of the device.
The HTCure™ laser manufacturing technology allows for a high precision mounting and ultra-stable permanent alignment of the output beams. A novel production process (patent pending) enables a beam overlap of better than 150 µrad. The optical platform can be customized for a higher degree of integration into the flow cytometer.
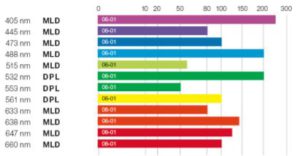
Through fully integrated electronics all lines can be individually controlled and intensity modulated. By combining both diode-pumped and direct diode laser technology, 12 different colors in the wavelength range 405 nm to 660 nm are available, with up to 100 mW output power per line.
Cobolt SkyraTM will facilitate reducing the size and cost of cytometers and other instruments for bioanalysis by removing the complexity of integrating multiple lasers and simplifying service and maintaince.
Cobolt Skyra™ is nearing market release (November). Cobolt in the process of CE certifying and gave a poster on the Skyra™ at this year’s CYTO flow cytometry conference in Boston, where it was well received and generated a fair bit of interest.
See the performance diagrams: SKYRA Poster